
Neu REFIX is one of the brands in the Nichi Glucan range of products; specifically, this is a produce of N-163 strain of Black Yeast Aureobasidium Pullulans, comes in a box of 20 sachet (each 8.0 gram of gel; 48mg Beta 1,3-1,6 glucan/Sachet)
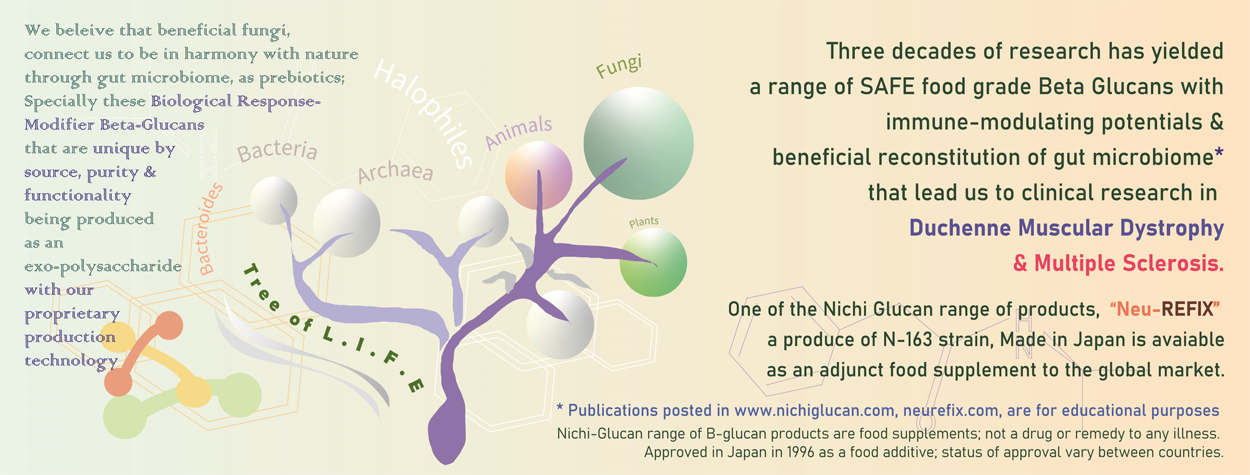
In pre-clinical studies*, in comparative studies in healthy volunteers, and in patients with Covid-19, the N-163 strain produce has been proven to have potent anti-inflammatory and anti-fibrotic properties. Based on such advantageous outcome, a clinical study in young boys with Duchenne Muscular Dystrophy (DMD) has been completed; a longer duration study under planning, while additional validations in pre-clinical MDX mice models under progress.
Publications:
![]() Raghavan K, Ikewaki N, Ichiyama K, Senthilkumar R, Preethy S, Abraham SJK. Reader Response: Microdystrophin Gene Therapy for Duchenne Muscular Dystrophy: The Ethics and Financial Toxicity of Exuberant Hope. Neurology. 2025;104(11):e213776. doi:10.1212/WNL.0000000000213776
Raghavan K, Ikewaki N, Ichiyama K, Senthilkumar R, Preethy S, Abraham SJK. Reader Response: Microdystrophin Gene Therapy for Duchenne Muscular Dystrophy: The Ethics and Financial Toxicity of Exuberant Hope. Neurology. 2025;104(11):e213776. doi:10.1212/WNL.0000000000213776
![]() Raghavan K, Dedeepiya VD, Yamamoto N, Ikewaki N, Iwasaki M, Dinassing A, Senthilkumar R, Preethy S, Abraham SJK. Randomised trial of Aureobasidium pullulans-produced beta 1,3-1,6-glucans in patients with Duchenne muscular dystrophy: Favourable changes in gut microbiota and clinical outcomes indicating their potential in epigenetic manipulation. BMJ Nutrition, Prevention & Health 2025;:bmjnph-2023-000776. doi:10.1136/bmjnph-2023-000776
Raghavan K, Dedeepiya VD, Yamamoto N, Ikewaki N, Iwasaki M, Dinassing A, Senthilkumar R, Preethy S, Abraham SJK. Randomised trial of Aureobasidium pullulans-produced beta 1,3-1,6-glucans in patients with Duchenne muscular dystrophy: Favourable changes in gut microbiota and clinical outcomes indicating their potential in epigenetic manipulation. BMJ Nutrition, Prevention & Health 2025;:bmjnph-2023-000776. doi:10.1136/bmjnph-2023-000776
![]() Raghavan K, Ikewaki N, Preethy S, Abraham SJK. Improving the therapeutic efficacy of gene therapy for Duchenne muscular dystrophy (DMD) by evaluating and managing inflammation. Front Genet. 2025 Jun 9;16:1569289. doi: 10.3389/fgene.2025.1569289.
Raghavan K, Ikewaki N, Preethy S, Abraham SJK. Improving the therapeutic efficacy of gene therapy for Duchenne muscular dystrophy (DMD) by evaluating and managing inflammation. Front Genet. 2025 Jun 9;16:1569289. doi: 10.3389/fgene.2025.1569289.
![]() Ikewaki N, Hegde ML, Rao KS, Raghavan K, Yamamoto N, Ichiyama K, Senthilkumar R, Preethy S, Abraham SJK. Approaching α-synuclein misfolding through gut microbiome; strategies for the management of neurological conditions. Future Neurol. 2025;20(1). doi: 10.1080/14796708.2025.2505398.
Ikewaki N, Hegde ML, Rao KS, Raghavan K, Yamamoto N, Ichiyama K, Senthilkumar R, Preethy S, Abraham SJK. Approaching α-synuclein misfolding through gut microbiome; strategies for the management of neurological conditions. Future Neurol. 2025;20(1). doi: 10.1080/14796708.2025.2505398.
![]() Pushkala S, Seshayyan S, Theranirajan E, Sudhakar D, Raghavan K, Dedeepiya VD, Ikewaki N, Iwasaki M, Preethy S, Abraham SJK. Efficient control of IL-6, CRP and ferritin in COVID-19 patients with two variants of beta-1,3-1,6 glucans in combination: An open-label, prospective, randomised clinical trial. Global Advances in Integrative Medicine and Health. 2025;14. doi:10.1177/27536130251327134
Pushkala S, Seshayyan S, Theranirajan E, Sudhakar D, Raghavan K, Dedeepiya VD, Ikewaki N, Iwasaki M, Preethy S, Abraham SJK. Efficient control of IL-6, CRP and ferritin in COVID-19 patients with two variants of beta-1,3-1,6 glucans in combination: An open-label, prospective, randomised clinical trial. Global Advances in Integrative Medicine and Health. 2025;14. doi:10.1177/27536130251327134
![]() Ikewaki N, Ichiyama K, Senthilkumar R, Preethy S, Abraham SJK. Significance of neutrophil to lymphocyte ratio (NLR) and gut microbiome of astronauts during space missions and suggestions for beneficial modulations using novel beta-glucans. Front Immunol. 2025;16:1538147. doi:10.3389/fimmu.2025.1538147
Ikewaki N, Ichiyama K, Senthilkumar R, Preethy S, Abraham SJK. Significance of neutrophil to lymphocyte ratio (NLR) and gut microbiome of astronauts during space missions and suggestions for beneficial modulations using novel beta-glucans. Front Immunol. 2025;16:1538147. doi:10.3389/fimmu.2025.1538147
![]() Preethy S, Sakamoto S, Higuchi T, Ichiyama K, Yamamoto N, Ikewaki N, Iwasaki M, Dedeepiya VD, Srinivasan S, Raghavan K, Rajmohan M, Senthilkumar R, Abraham SJK. Enhanced expression of dystrophin, IGF-1, CD44 and MYH3 in plasma and skeletal muscles including diaphragm of mdx mice after oral administration of Neu REFIX beta 1,3-1,6 glucan. Sci Rep. 2025;15:7232. doi:10.1038/s41598-025-07232-4.
Preethy S, Sakamoto S, Higuchi T, Ichiyama K, Yamamoto N, Ikewaki N, Iwasaki M, Dedeepiya VD, Srinivasan S, Raghavan K, Rajmohan M, Senthilkumar R, Abraham SJK. Enhanced expression of dystrophin, IGF-1, CD44 and MYH3 in plasma and skeletal muscles including diaphragm of mdx mice after oral administration of Neu REFIX beta 1,3-1,6 glucan. Sci Rep. 2025;15:7232. doi:10.1038/s41598-025-07232-4.
![]() Preethy S, Aoki Y, Minegishi K, Iwasaki M, Senthilkumar R, Abraham S. Resolution of fibrosis in mdx dystrophic mouse after oral consumption of N-163 strain of Aureobasidium pullulans produced β-glucan. Sci Rep 13, 17008 (2023). doi: 10.1038/s41598-023-44330-0
Preethy S, Aoki Y, Minegishi K, Iwasaki M, Senthilkumar R, Abraham S. Resolution of fibrosis in mdx dystrophic mouse after oral consumption of N-163 strain of Aureobasidium pullulans produced β-glucan. Sci Rep 13, 17008 (2023). doi: 10.1038/s41598-023-44330-0
![]() Senthilkumar Preethy, Naoki Yamamoto, Kottoorathu Mammen Cherian, Rajasekaran Premsekar, Gary A Levy, Rajappa Senthilkumar, Samuel JK Abraham. Reduction in myocardial fibrosis in MDX mice on oral consumption of Aureobasidium Pullulans produced Neu REFIX Beta-glucans; holds potential as an adjuvant in managing post-transplantation organ fibrosis. biorxiv: 10.1101/2023.09.25.559276v1. doi: 10.1101/2023.09.25.559276 (In pre-print)
Senthilkumar Preethy, Naoki Yamamoto, Kottoorathu Mammen Cherian, Rajasekaran Premsekar, Gary A Levy, Rajappa Senthilkumar, Samuel JK Abraham. Reduction in myocardial fibrosis in MDX mice on oral consumption of Aureobasidium Pullulans produced Neu REFIX Beta-glucans; holds potential as an adjuvant in managing post-transplantation organ fibrosis. biorxiv: 10.1101/2023.09.25.559276v1. doi: 10.1101/2023.09.25.559276 (In pre-print)
![]() Raghavan K, Dedeepiya VD, Srinivasan S, Pushkala S, Subramanian S, Ikewaki N, Iwasaki M, Senthilkumar R, Preethy S, Abraham S. Beneficial immune-modulatory effects of the N-163 strain of Aureobasidium pullulans-produced 1,3-1,6 Beta glucans in Duchenne muscular dystrophy: Results of an open-label, prospective, exploratory case-control clinical study. IBRO Neuroscience reports 2023; 15: 90-99. doi: 10.1016/j.ibneur.2023.06.007.
Raghavan K, Dedeepiya VD, Srinivasan S, Pushkala S, Subramanian S, Ikewaki N, Iwasaki M, Senthilkumar R, Preethy S, Abraham S. Beneficial immune-modulatory effects of the N-163 strain of Aureobasidium pullulans-produced 1,3-1,6 Beta glucans in Duchenne muscular dystrophy: Results of an open-label, prospective, exploratory case-control clinical study. IBRO Neuroscience reports 2023; 15: 90-99. doi: 10.1016/j.ibneur.2023.06.007.
![]() Ikewaki N, Sonoda T, Kurosawa G, Iwasaki M, Dedeepiya VD, Senthilkumar R, Preethy S, Abraham S . Beta 1,3-1,6 glucans produced by two novel strains of Aureobasidium pullulans exert immune and metabolic beneficial effects in healthy middle-aged Japanese men: Results of an exploratory randomized control study. J Aging Res & Lifestyle 2023;12:61-71. doi: 10.14283/jarlife.2023.11.
Ikewaki N, Sonoda T, Kurosawa G, Iwasaki M, Dedeepiya VD, Senthilkumar R, Preethy S, Abraham S . Beta 1,3-1,6 glucans produced by two novel strains of Aureobasidium pullulans exert immune and metabolic beneficial effects in healthy middle-aged Japanese men: Results of an exploratory randomized control study. J Aging Res & Lifestyle 2023;12:61-71. doi: 10.14283/jarlife.2023.11.
![]() Kadalraja Raghavan, Thanasekar Sivakumar, Koji Ichiyama, Naoki Yamamoto, Mangaleswaran Balamurugan, Vidyasagar Devaprasad Dedeepiya, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel JK Abraham. Efficacy of N-163 beta-glucan in beneficially improving biomarkers of relevance to muscle function in patients with muscular dystrophies in a pilot clinical study. medrxiv 2023.07.20.23292982v1; doi : 10.1101/2023.07.20.23292982.(In pre-print)
Kadalraja Raghavan, Thanasekar Sivakumar, Koji Ichiyama, Naoki Yamamoto, Mangaleswaran Balamurugan, Vidyasagar Devaprasad Dedeepiya, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel JK Abraham. Efficacy of N-163 beta-glucan in beneficially improving biomarkers of relevance to muscle function in patients with muscular dystrophies in a pilot clinical study. medrxiv 2023.07.20.23292982v1; doi : 10.1101/2023.07.20.23292982.(In pre-print)
![]() Kadalraja Raghavan, Vidyasagar Devaprasad Dedeepiya, Subramaniam Srinivasan, Subramanian Pushkala, Sudhakar S. Bharatidasan, Nobunao Ikewaki, Masaru Iwasaki, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel J.K. Abraham. Beneficial immune-modulatory effects of the N-163 strain of Aureobasidium pullulans-produced 1,3-1,6 Beta glucans in Duchenne muscular dystrophy: Results of an open-label, prospective, exploratory case-control clinical study. IBRO Neuroscience Reports 15 (2023) 90-99; doi : 10.1016/j.ibneur.2023.06.007.
Kadalraja Raghavan, Vidyasagar Devaprasad Dedeepiya, Subramaniam Srinivasan, Subramanian Pushkala, Sudhakar S. Bharatidasan, Nobunao Ikewaki, Masaru Iwasaki, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel J.K. Abraham. Beneficial immune-modulatory effects of the N-163 strain of Aureobasidium pullulans-produced 1,3-1,6 Beta glucans in Duchenne muscular dystrophy: Results of an open-label, prospective, exploratory case-control clinical study. IBRO Neuroscience Reports 15 (2023) 90-99; doi : 10.1016/j.ibneur.2023.06.007.
![]() Senthilkumar Preethy, Shuji Sakamoto, Takuma Higuchi, Koji Ichiyama, Naoki Yamamoto, Nobunao Ikewaki, Masaru Iwasaki, Vidyasagar Devaprasad Dedeepiya, Subramaniam Srinivasan, Mathaiyan Rajmohan, Rajappa Senthilkumar, Samuel JK Abraham. Enhanced muscle regeneration in mdx mice, Duchenne muscular dystrophy animal model, proven by CD44 & MYH3 expression, on oral feeding of N-163 strain of Aureobasidium Pullulans produced B-Glucan. biorxiv 2023.06.06.543858v1 doi: 10.1101/2023.06.06.543858 (In pre-print)
Senthilkumar Preethy, Shuji Sakamoto, Takuma Higuchi, Koji Ichiyama, Naoki Yamamoto, Nobunao Ikewaki, Masaru Iwasaki, Vidyasagar Devaprasad Dedeepiya, Subramaniam Srinivasan, Mathaiyan Rajmohan, Rajappa Senthilkumar, Samuel JK Abraham. Enhanced muscle regeneration in mdx mice, Duchenne muscular dystrophy animal model, proven by CD44 & MYH3 expression, on oral feeding of N-163 strain of Aureobasidium Pullulans produced B-Glucan. biorxiv 2023.06.06.543858v1 doi: 10.1101/2023.06.06.543858 (In pre-print)
![]() Vidyasagar Devaprasad Dedeepiya, Chockanathan Vetrievel, Nobunao Ikewaki, Koji Ichiyama, Naoki Yamamoto, Hiroto Kawashima, Sudhakar S Bharatidasan, Subramaniam Srinivasan, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel JK Abraham. Improvement in Expanded Disability Status Scale (EDSS) and anti-inflammatory parameters in patients with multiple sclerosis following oral consumption of N-163 strain of Aureobasidium pullulans produced beta glucan in a pilot clinical study. medRxiv 2023.05.14.23289953v2 doi: 10.1101/2023.05.14.23289953 (In pre-print)
Vidyasagar Devaprasad Dedeepiya, Chockanathan Vetrievel, Nobunao Ikewaki, Koji Ichiyama, Naoki Yamamoto, Hiroto Kawashima, Sudhakar S Bharatidasan, Subramaniam Srinivasan, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel JK Abraham. Improvement in Expanded Disability Status Scale (EDSS) and anti-inflammatory parameters in patients with multiple sclerosis following oral consumption of N-163 strain of Aureobasidium pullulans produced beta glucan in a pilot clinical study. medRxiv 2023.05.14.23289953v2 doi: 10.1101/2023.05.14.23289953 (In pre-print)
![]() Kadalraja Raghavan, Thanasekar Sivakumar, Sudhakar S Bharatidasan, Subramaniam Srinivasan, Vidyasagar Devaprasad Dedeepiya, Nobunao Ikewaki, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel JK Abraham. Efficacy of N-163 strain of Aureobasidium pullulans-produced beta-glucan in improving muscle strength and function in patients with Duchenne muscular dystrophy; Results of a 6-month non-randomised open-label linear clinical trial. medRxiv 2023.04.29.23289260v1; doi: 10.1101/2023.04.29.23289260 (In pre-print)
Kadalraja Raghavan, Thanasekar Sivakumar, Sudhakar S Bharatidasan, Subramaniam Srinivasan, Vidyasagar Devaprasad Dedeepiya, Nobunao Ikewaki, Rajappa Senthilkumar, Senthilkumar Preethy, Samuel JK Abraham. Efficacy of N-163 strain of Aureobasidium pullulans-produced beta-glucan in improving muscle strength and function in patients with Duchenne muscular dystrophy; Results of a 6-month non-randomised open-label linear clinical trial. medRxiv 2023.04.29.23289260v1; doi: 10.1101/2023.04.29.23289260 (In pre-print)
![]() Raghavan K, Dedeepiya VD, Yamamoto N, Ikewaki N, Iwasaki M, Dinassing A, Senthilkumar R, Preethy S, Abraham SJK. Randomised trial of Aureobasidium pullulans-produced beta 1,3-1,6-glucans in patients with Duchenne muscular dystrophy: Favourable changes in gut microbiota and clinical outcomes indicating their potential in epigenetic manipulation. medRxiv 2022.12.09.22283273; doi: 10.1101/2022.12.09.22283273 (In pre-print)
Raghavan K, Dedeepiya VD, Yamamoto N, Ikewaki N, Iwasaki M, Dinassing A, Senthilkumar R, Preethy S, Abraham SJK. Randomised trial of Aureobasidium pullulans-produced beta 1,3-1,6-glucans in patients with Duchenne muscular dystrophy: Favourable changes in gut microbiota and clinical outcomes indicating their potential in epigenetic manipulation. medRxiv 2022.12.09.22283273; doi: 10.1101/2022.12.09.22283273 (In pre-print)
![]() Raghavan K, Dedeepiya VD, Srinivasan S, Pushkala S, Subramanian S, Ikewaki N, Iwasaki M, Senthilkumar R, Preethy S, Abraham S. Disease-modifying immune-modulatory effects of the N-163 strain of Aureobasidium pullulans-produced 1,3-1,6 Beta glucans in young boys with Duchenne muscular dystrophy: Results of an open-label, prospective, randomized, comparative clinical study medRxiv 10.1101/2021.12.13.21267706v1; doi: 10.1101/2021.12.13.21267706 (In pre-print)
Raghavan K, Dedeepiya VD, Srinivasan S, Pushkala S, Subramanian S, Ikewaki N, Iwasaki M, Senthilkumar R, Preethy S, Abraham S. Disease-modifying immune-modulatory effects of the N-163 strain of Aureobasidium pullulans-produced 1,3-1,6 Beta glucans in young boys with Duchenne muscular dystrophy: Results of an open-label, prospective, randomized, comparative clinical study medRxiv 10.1101/2021.12.13.21267706v1; doi: 10.1101/2021.12.13.21267706 (In pre-print)
Conference Presentations:
![]() 16~19, May 2024
: Abraham S et al. Novel B-glucan based disease modifying treatment adjuvant in Duchenne muscular dystrophy.Concurrent Sessions: Duchenne muscular dystrophy. MENA Congress for Rare Diseases. Abu Dhabi, UAE.
16~19, May 2024
: Abraham S et al. Novel B-glucan based disease modifying treatment adjuvant in Duchenne muscular dystrophy.Concurrent Sessions: Duchenne muscular dystrophy. MENA Congress for Rare Diseases. Abu Dhabi, UAE.
![]() 27~29, June 2024
: Abraham S, Thadeus J, Santhiya Vadhana A, Suresh Durai J, James Joshuva T, Miura I, Yamamoto N, Ichiyama K, Preethy S, Senthilkumar R . Aureobasidium pullulans produced Beta-1,3-1,6 glucans improving clinical parameters, ameliorating inflammation and
skin lymphocyte infiltration in patients with Psoriasis vulgaris. 7th IFPA Conference (World Psoriasis & Psoriatic Arthritis Conference 2024. Stockholm, Sweden.
27~29, June 2024
: Abraham S, Thadeus J, Santhiya Vadhana A, Suresh Durai J, James Joshuva T, Miura I, Yamamoto N, Ichiyama K, Preethy S, Senthilkumar R . Aureobasidium pullulans produced Beta-1,3-1,6 glucans improving clinical parameters, ameliorating inflammation and
skin lymphocyte infiltration in patients with Psoriasis vulgaris. 7th IFPA Conference (World Psoriasis & Psoriatic Arthritis Conference 2024. Stockholm, Sweden.
![]() 3~6, March 2024
: Abraham S, Aoki Y, Minegishi K, Ichiyama K, Senthilkumar R, Preethy S. Raghavan K, Ikewaki N, Iwasaki M. Decline in muscle fatigue score of mdx mice after oral administration of Neu-REFIX Beta 1,3-1,6 Glucans in a short duration study of 45 days. MDA Conference 2024, USA.
3~6, March 2024
: Abraham S, Aoki Y, Minegishi K, Ichiyama K, Senthilkumar R, Preethy S. Raghavan K, Ikewaki N, Iwasaki M. Decline in muscle fatigue score of mdx mice after oral administration of Neu-REFIX Beta 1,3-1,6 Glucans in a short duration study of 45 days. MDA Conference 2024, USA.
![]() 3~6, March 2024
: Abraham S, Sakamoto S, Higuchi T, Aoki Y, Minegishi K, Ikewaki N, Ichiyama K, Raghavan K, Senthilkumar R, Preethy S, Iwasaki M. Evaluation of infiltrating CD68 macrophages and myofiber cross-sectional area in the skeletal muscle of mdx mice after oral administration of Neu REFIX. Late breaking abstracts. MDA Conference 2024, USA.
3~6, March 2024
: Abraham S, Sakamoto S, Higuchi T, Aoki Y, Minegishi K, Ikewaki N, Ichiyama K, Raghavan K, Senthilkumar R, Preethy S, Iwasaki M. Evaluation of infiltrating CD68 macrophages and myofiber cross-sectional area in the skeletal muscle of mdx mice after oral administration of Neu REFIX. Late breaking abstracts. MDA Conference 2024, USA.
![]() 15~19, October 2023
: Abraham S et al. Beneficial changes of gut microbiome commonly relevant to multiple sclerosis and Duchenne muscular dystrophy (DMD) after consumption of A.pullulansN-163-B-glucan –Results from clinical studies XXVI World Congress of Neurology (WCN2023), Montreal, Canada.
15~19, October 2023
: Abraham S et al. Beneficial changes of gut microbiome commonly relevant to multiple sclerosis and Duchenne muscular dystrophy (DMD) after consumption of A.pullulansN-163-B-glucan –Results from clinical studies XXVI World Congress of Neurology (WCN2023), Montreal, Canada.
![]() 3~7, October 2023
: Samuel JK Abraham, Gary A Levy, Naoki Yamamoto, Kottoorathu Mammen Cherian, Rajasekaran Premsekar, Rajappa Senthilkumar, Senthilkumar Preethy. Amelioration of myocardial fibrosis in mdx mice of Duchenne muscular dystrophy (DMD) on oral consumption of Aureobasidium Pullulans produced Neu REFIX Beta glucans. World Muscle Society, WMS2023, 3~7, October 2023, Charleston, USA.
3~7, October 2023
: Samuel JK Abraham, Gary A Levy, Naoki Yamamoto, Kottoorathu Mammen Cherian, Rajasekaran Premsekar, Rajappa Senthilkumar, Senthilkumar Preethy. Amelioration of myocardial fibrosis in mdx mice of Duchenne muscular dystrophy (DMD) on oral consumption of Aureobasidium Pullulans produced Neu REFIX Beta glucans. World Muscle Society, WMS2023, 3~7, October 2023, Charleston, USA.
![]() Samuel JK Abraham, Kadalraja Raghavan, Thanasekar Sivakumar, Sudhakar S Bharathidasan, Subramaniam Srinivasan, Vidyasagar Devaprasad Dedeepiya, Nobunao Ikewaki, Masaru Iwasaki, Rajappa Senthilkumar, Senthilkumar Preethy. Efficacy of a safe ß-glucan in improving muscle function in Duchenne Muscular Dystrophy; results of a 6-month nonrandomized open-label extension trial. MDA Conference 2023.
Samuel JK Abraham, Kadalraja Raghavan, Thanasekar Sivakumar, Sudhakar S Bharathidasan, Subramaniam Srinivasan, Vidyasagar Devaprasad Dedeepiya, Nobunao Ikewaki, Masaru Iwasaki, Rajappa Senthilkumar, Senthilkumar Preethy. Efficacy of a safe ß-glucan in improving muscle function in Duchenne Muscular Dystrophy; results of a 6-month nonrandomized open-label extension trial. MDA Conference 2023.
![]() Samuel JK Abraham, Shuji Sakamoto, Takuma Higuchi, Subramaniam Srinivasan, Vidyasagar Devaprasad Dedeepiya, Nobunao Ikewaki, Masaru Iwasaki, Mathaiyan Rajmohan, Rajappa Senthilkumar, Senthilkumar Preethy. Enhanced muscle regeneration in MDX Duchenne muscular dystrophy-mice, proven by CD44 & MYH3, on consumption of A.Pullulans produced Neu REFIX ß-Glucan.
MDA Conference 2023.
Samuel JK Abraham, Shuji Sakamoto, Takuma Higuchi, Subramaniam Srinivasan, Vidyasagar Devaprasad Dedeepiya, Nobunao Ikewaki, Masaru Iwasaki, Mathaiyan Rajmohan, Rajappa Senthilkumar, Senthilkumar Preethy. Enhanced muscle regeneration in MDX Duchenne muscular dystrophy-mice, proven by CD44 & MYH3, on consumption of A.Pullulans produced Neu REFIX ß-Glucan.
MDA Conference 2023.
* Links to publications are posted for educational purposes only.
Neu REFIX is a food supplement, not a drug or substitute to any drug or remedy to illness. No therapeutic claims; those who want to discuss about the clinical studies reported, may consult a physician.
Nichi Glucan range of products available in the brands, viz., Nichi Glucan, Nichi Glucan REFIX, Neu-REFIX, Nichi BRITE, and Nichi GLOW are free from any of the following notified common allergens listed as per the Consumer Affairs Agency, Government of Japan. (Japanese)
Shrimp, crab, wheat, buckwheat, egg, milk, peanut (peanuts), Almond, abalone, squid, salmon roe, orange, cashew nut, kiwi fruit, beef, walnut, sesame, salmon, mackerel, soybean, chicken, banana, pork, matsutake mushroom, peach, yam, apple, gelatin.
Disclaimer


